MK677 (Ibutamoren): The Complete Guide
*DISCLAIMER: Selective Androgen Receptor Modulators (SARMs) are not approved by the FDA for any currently acceptable medicinal/therapeutic purpose. Therefore, this article does not serve as medical advice and is not to be intended to be used to diagnose, treat, cure or prevent any disease. SARMS are to be used for research purposes only and not intended for human consumption.*
What is MK677?
Although MK677 (also known as Ibutamoren) is commonly grouped alongside other known SARMs such as LGD-4033 and RAD140, it’s not actually a SARM at all. MK677 is a growth hormone (GH) secretagogue and ghrelin receptor agonist. In other words, it promotes the release and output of the hormones GH and ghrelin, the latter of which stimulates the release of GH. 
MK677 was initially developed as a means to combat GH deficiency syndrome [1]. It’s also been investigated for its ability to combat frailty in the elderly [2]. But just like SARMs, MK677 is considered an “Investigational New Drug” by the Food & Drug Administration (FDA). This means that it hasn’t yet been approved for human use, despite a large bulk of its research having been carried out in the mid-late 1990s.
Growth Hormone (GH)
In adults, GH has a variety of functions. Some that are of interest to those in the fitness/bodybuilding communities include:
- Bone formation
- GH plays a vital role in the formation and maintenance of bone tissue via calcium retention [3].
- Protein synthesis
- GH increases muscle mass via sarcomere hypertrophy [4]
- Increases rates of lipolysis (breakdown of fatty acids) [5]
Mechanism of Action
So why would somebody take a GH secretagogue such as MK677 in the first place? Wouldn’t it be easier to simply take GH instead?
What turns most people away from taking GH directly is perhaps that it’s incredibly expensive, especially for a drug that requires daily administration. In fact, It’s not unheard of for pharmaceutical grade GH to cost as much as $1,000-$5,000 per month! So just imagine how much it would cost somebody to use it for recreational purposes, which typically necessitates the use of much higher dosages.
Exorbitantly high prices may make the majority of recreational users think about making their GH purchase(s) on the black market. However, doing so carries with it an enormous amount of risk. GH is widely faked on the black market due to its high cost. So it’s highly recommended that you avoid going this route.
Another reason why people choose not to take GH directly is because it’s not able to be administered orally due to its large molecular structure.
So any person who wishes to take GH must be willing to perform either intramuscular (muscle) or subcutaneous (fat) injections on a daily basis. Some protocols even call for multiple injections daily.
MK677, on the other hand, has a much smaller structure in comparison to both GH itself as well as other GH-releasing peptides (GHRPs). Peptides are significantly large structures and although they can be made into drugs, they often can’t be administered via oral route. On the other hand, smaller molecule secretagogues such as MK677 are classified as non-peptide agonists of GH. Therefore, they have the ability to be administered orally. This also allows for MK677 to remain stable at room temperature, thus not necessitating any special storage requirements.
Ghrelin
MK677’s functions to stimulate the action of a hormone called ghrelin. Ghrelin is often referred to informally as the “hunger hormone”, since its levels are highest when hungry, initially stimulating the individual to find and eat food [6]. Ghrelin levels are highest between meal times and after waking.
Ghrelin stimulates GH release from the anterior pituitary gland in the brain [7]. It’s been shown to reliably induce hunger, regardless of body composition or level of body fatness. This includes lean, obese, and even malnourished individuals [8].
Not only does it have effects pertaining directly to food intake, it also regulates body weight, glucose metabolism, gut motility, circadian rhythm (sleep), taste, stress/anxiety regulation, and even prevents muscular atrophy [9,10,11,12,13,14].
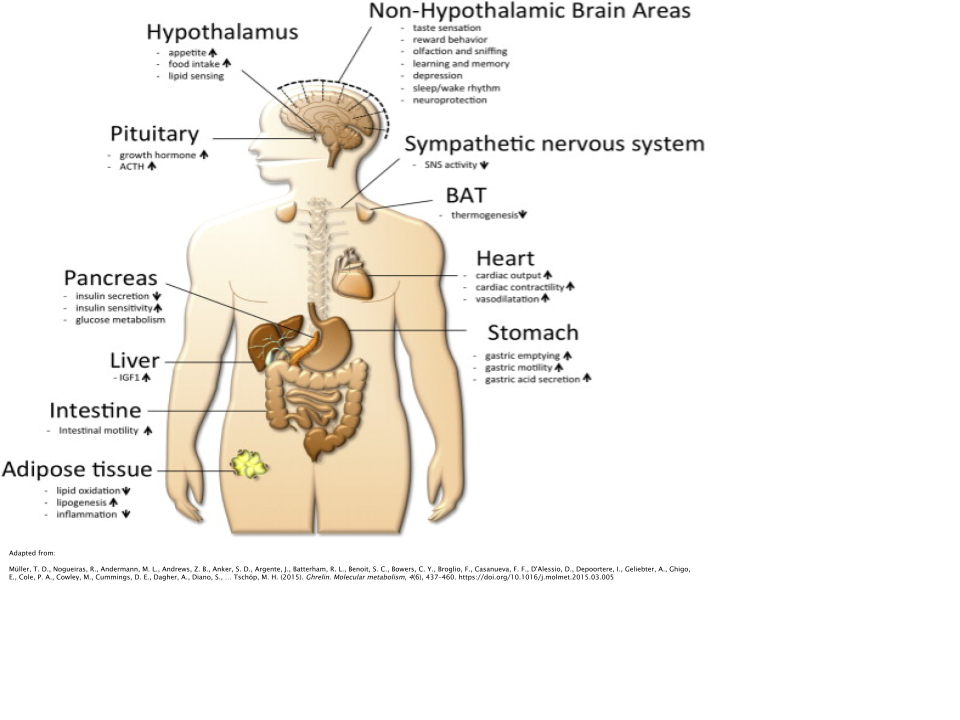
Ghrelin is needed in order for GH to be secreted. Clinical trials have shown that either a single injection or 24-hour infusion of ghrelin induces both the acute release of GH, as well as pulsatile GH secretion over a 24-hour period [15,16,17].
Animal Data
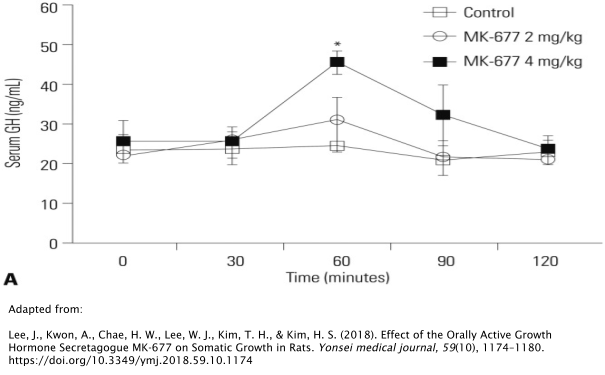
Oral administration of 4mg/kg/day of MK677 substantially increases concentrations of GH by nearly double 1 hour post-administration, as demonstrated in rat models [18]. In beagles, this rise in GH has been shown to last for up to 6 hours after a single dose [19]. However, GH levels returned to baseline levels in both of these studies shortly afterward.
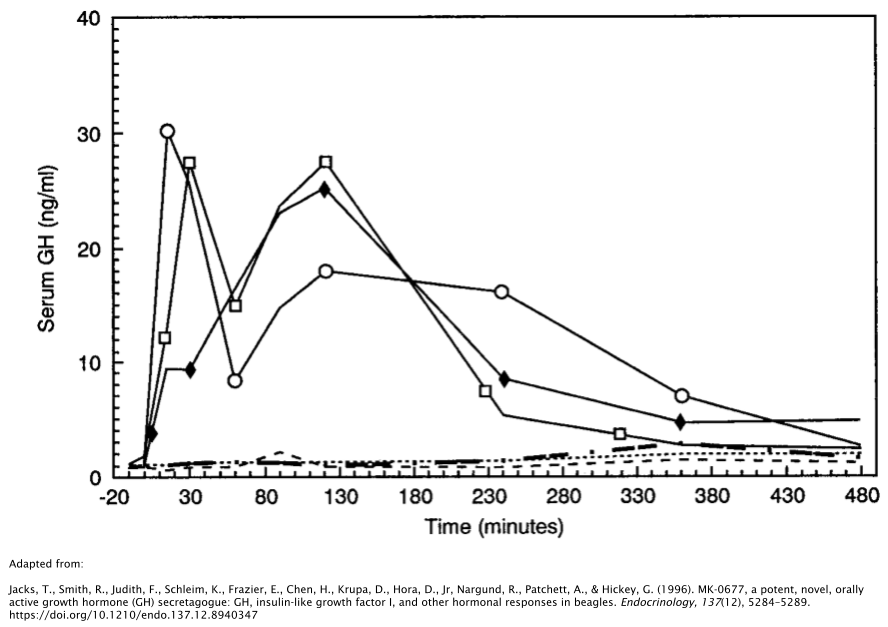
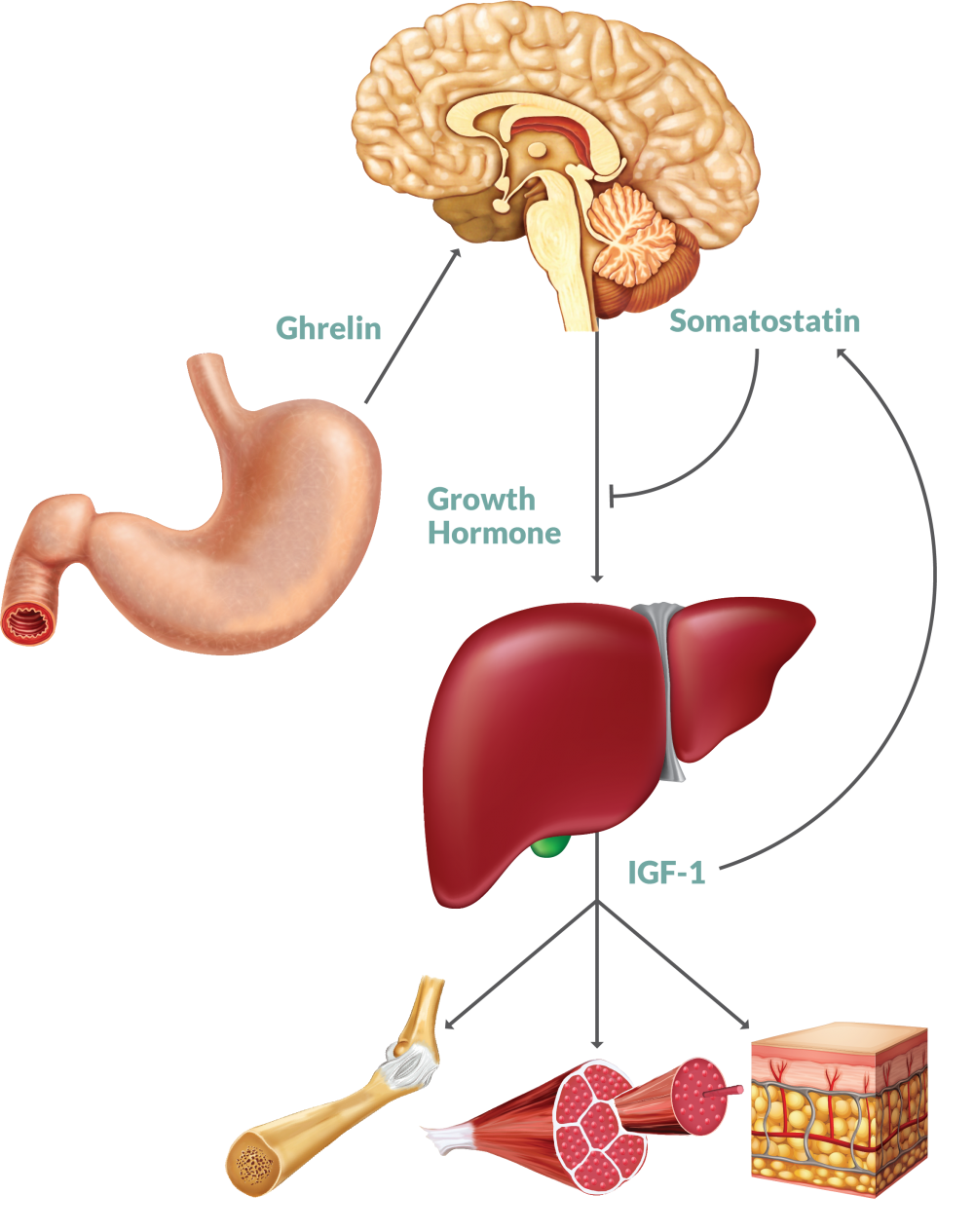
Insulin-Like Growth Factor-1 (IGF-1)
Results on MK677s effects on Insulin-Like Growth Factor-1 (IGF-1) are mixed.
IGF-1 is structurally similar to insulin and its production in the liver is stimulated by GH. After IGF-1 production is initiated, it begins to promote cellular growth on various tissue types in the body, namely skeletal muscle, bone, cartilage, and liver [20]. IGF-1 controls macronutrient metabolism (protein, carbs, fats) in the various cells it has influence over. This, in turn, increases the production of proteins within these cells [21,22].
The aforementioned rat study showed no effect on IGF-1.
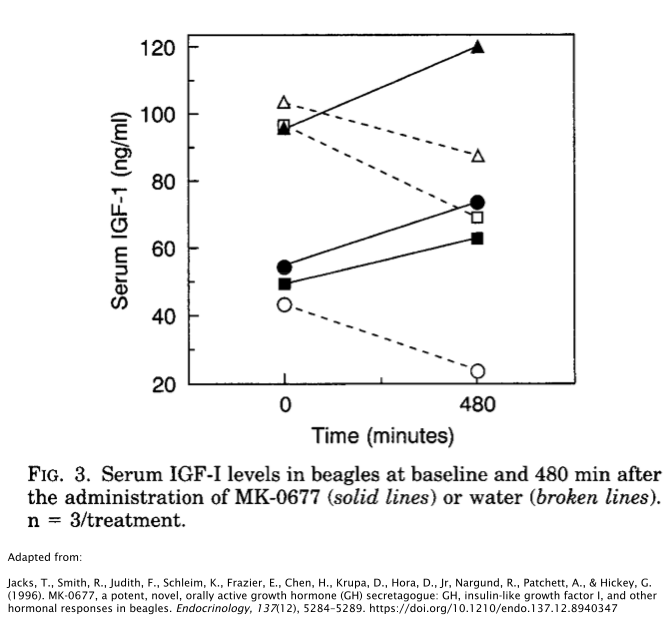
On the other hand, the beagle study showed a significant increase in IGF-1 compared to the placebo. This is quite a strange phenomenon theoretically speaking, as the rats were technically given a higher dose than the beagles; 4mg/kg/day vs. 1mg/kg/day, respectively. More research is needed to delve into the mechanism behind this occurrence.
Desensitization
While the administration of MK677 was able to initially stimulate GH secretion in the rats, prolonged administration of MK677 after 6 weeks didn’t promote any additional tissue growth. In fact, it even abolished the GH-stimulatory effect of MK677. The researchers hypothesized that this may have been due to desentization of GHRPs on GH secretion, which has been reported on numerous occasions in GHRP research [17,23].
In one study using growth-retarded rats, infusion of growth hormone-releasing peptide 6 (GHRP-6) was only effective in accelerating growth for the first 2 days of infusion. This effect essentially plummeted after those initial 2 days [23].
A study conducted in cell culture confirmed this phenomenon, as repeated exposure of GHRP-6 induced desensitization of GH release from pituitary cells [24].
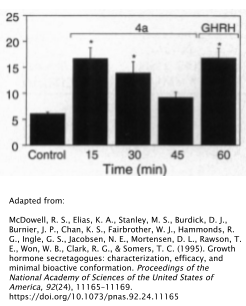
Human Data
In one study involving 9 young, healthy men split amongst 3 groups, each group was assigned to a different dosage amount of MK677; placebo (0mg/day), 5mg/day, or 25mg/day of MK677 before bedtime for 1 week [25]. This study was designed as a crossover, meaning that each group cycled through each of the treatment conditions one time. The following illustration shows a schematic of what this research design commonly looks like with 2 treatment groups.
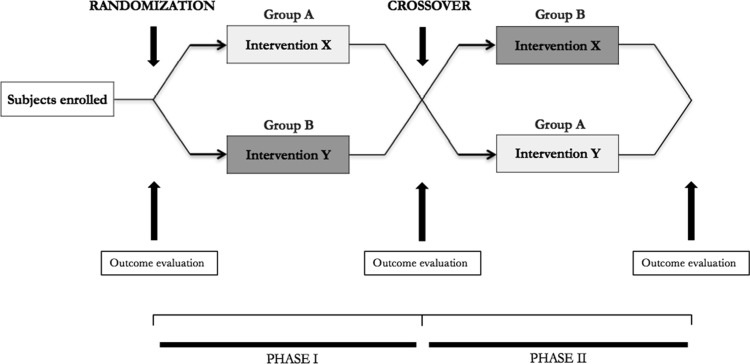
The results of studies like these are sure to be made accurate through what researchers call a “washout” period, where the drug is allowed to clear the subjects’ systems before they switch to a different treatment condition. Studies of this type of design take longer amounts of time to carry out and are often more expensive. Therefore, they are very valuable when they are able to be carried out properly in comparison to other types of study designs, particularly when given the fact that the aforementioned study at hand used 3 treatment groups. So it’s actually somewhat more complicated than what is illustrated by the schematic.
Going back to the study results themselves, what the researchers found was that MK677 increased GH pulse frequency along with plasma levels of IGF-1 in a dose-dependent manner, meaning that the higher the dose of MK677, the higher the plasma levels of IGF-1.
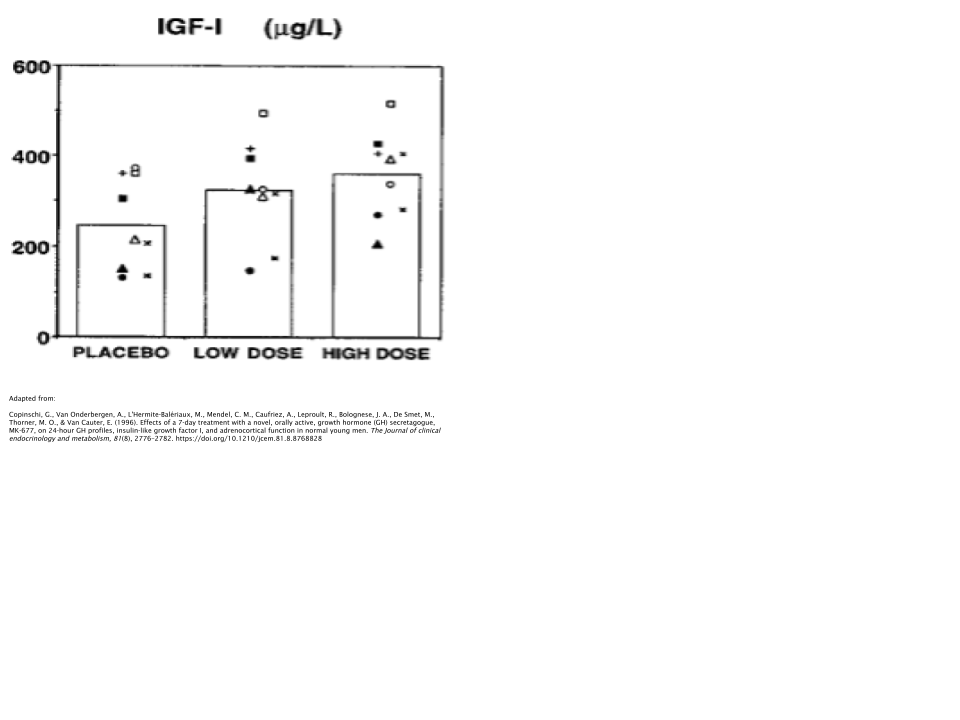
Body Fat
Obesity is well-known to cause impaired secretion of GH [26]. In fact, obesity and GH deficiency share more in common than one might imagine. This includes insulin resistance, greater levels of body fat, and increased cardiovascular mortality rate [27,28].
In one study, 24 obese subjects took either 25mg/day of MK677 or placebo over the course of 2 months [29]. Researchers discovered that those who took MK677 increased their IGF-1 levels by ~40%, along with the increase of Insulin-Like Growth Factor Binding Protein-3 (IGFBP-3), which is responsible for ~80% of all IGF-1 binding [30].
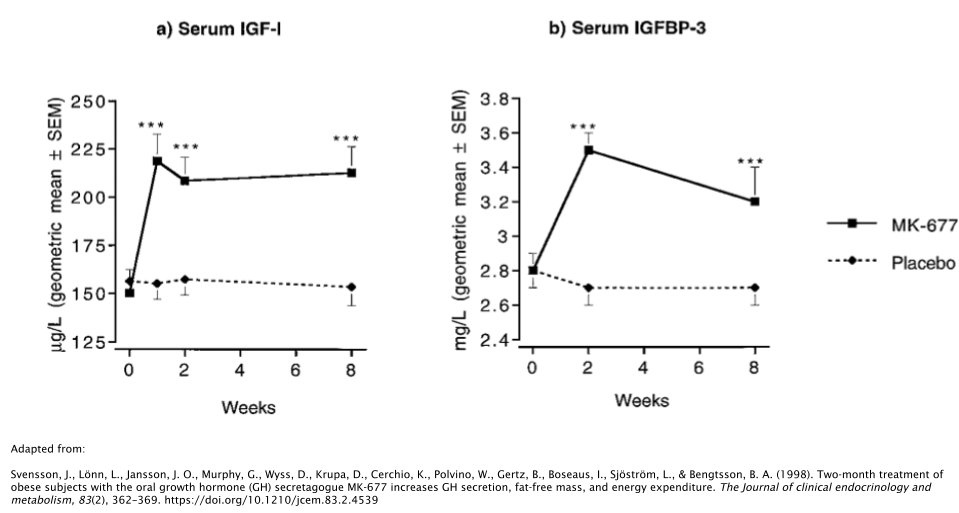
The fat-free mass of the subjects increased as well, whereas no effects were seen on body fat levels. It should be noted that “fat-free mass”, when used as a measure in the scientific literature, simply means that it’s a measure of all tissues in the body with the exception of adipose (fat). So this would include tissues such as muscle, bone, organs, and connective tissues.
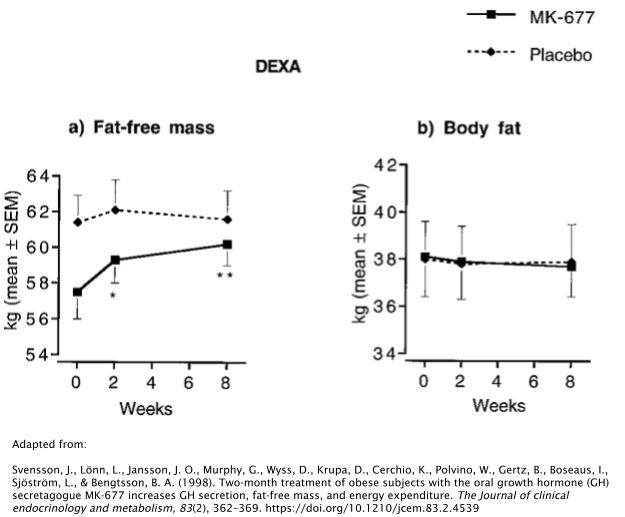
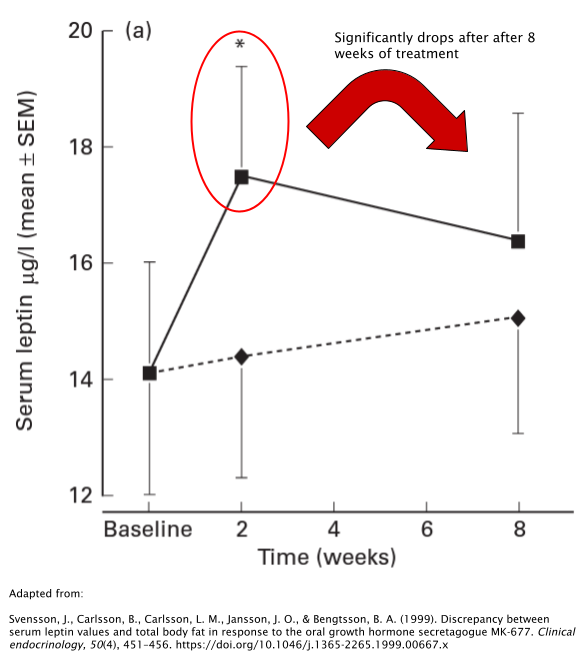
It’s unclear whether or not MK677 has the ability to alter body fat levels directly. Another study involving obese subjects also reported no significant changes in body fat [31]. However, serum levels of leptin (the opposing hormone to ghrelin) were significantly higher after 2 weeks of treatment with 25mg/day of MK677. Interestingly, however, these results seem to drastically fade as the treatment continues, with leptin levels decreasing dramatically between 2 and 8 weeks of treatment.
While it seems promising that MK677 has the potential to have a favorable influence over body fat levels, the research has failed to find any direct causal link thus far.
Cortisol
Prolonged use of GHRPs are often associated with a rise in cortisol [32]. The aforementioned study involving the obese subjects discovered that MK677 doesn’t in fact have a significant effect on cortisol like other GHRP’s.
While the initial dose of MK677 caused an increase of cortisol, it dissipated after analysis was conducted at both the 2 and 8 week timepoints. This is important from a clinical perspective, as obesity is often associated with hypercortisolism (Cushing’s syndrome); a chronically elevated level of cortisol that requires medical attention [33].
Bone Density
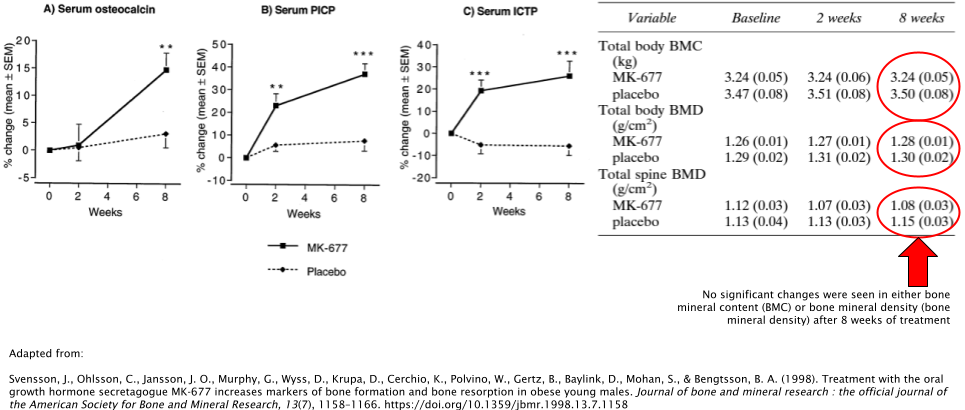
MK677 has also demonstrated its ability to favorably increase markers of bone formation when used at doses of 10mg/day and 25mg/day [34,35]. While this is a good sign that MK677 has the potential to be used for bone degradation-related conditions such as osteoporosis, future research is needed to determine whether or not this positively affects bone mineral density (BMD) and bone mineral content (BMC), as they failed to show any significant improvements compared to the placebo group in one of the studies. In the other study, both BMC and BMD were not directly measured whatsoever.
Sleep Quality
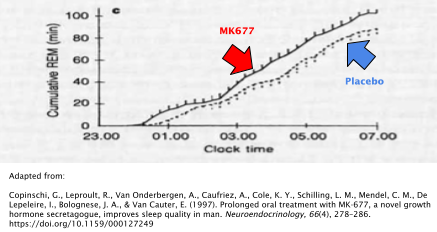
When taken at a dose of 25mg before bedtime each night, MK677 improves the quality of sleep in both young and old individuals [36]. Rapid-eye-movement (REM) sleep was the primary variable that was observed in the study that examined this phenomenon. REM is a deep stage of sleep where vivid dreams often occur and has been associated with its importance in memory maintenance and formation [37].
Young subjects experienced a 20% increase in REM sleep compared to placebo while older adults experienced a ~50% increase in REM sleep.
Alzheimer’s Disease
Research has even gone so far to investigate MK677’s potential use in the treatment of Alzheimer’s Disease (AD), interestingly enough.
The most significant pathological indicator of AD is β-amyloid; a group of peptides that are present in high amounts in those with AD [38]. One hypothesis that lies behind the treatment of AD is to clear as much β-amyloid from the brain as possible. One way in which this can occur is to increase IGF-1 levels, particularly since IGF-1 levels decline with age, aggravating the situation even more so.
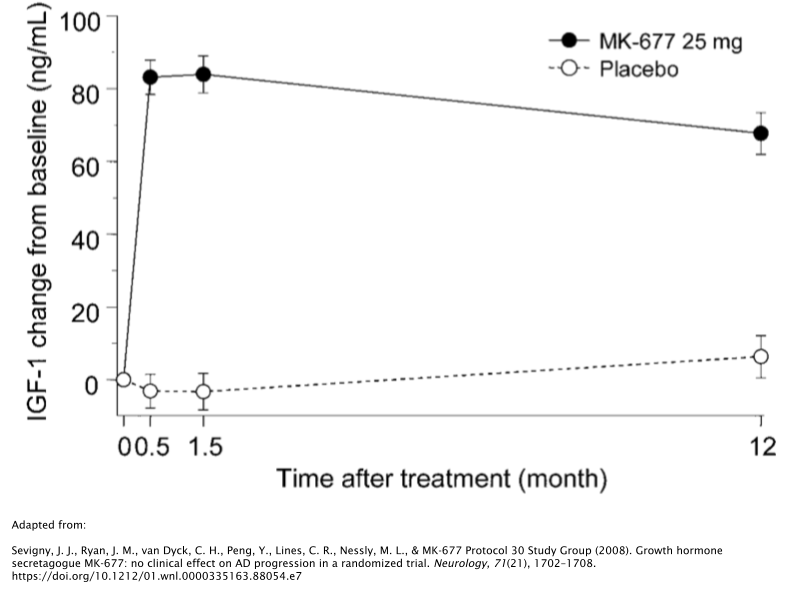
While 12 months of treatment with 25mg/day of MK677 caused a marked increase of IGF-1 levels compared to the patients baseline levels, patients failed to show improvements in the symptoms associated with their AD [39].
Bioavailability
MK677 has remarkably impressive bioavailability, provided that it’s an orally-administered drug. Rates of bioavailability in humans have been estimated to be as high as >60% [40]. This is significantly higher than similar drugs such as GHRP-6, which has a much poorer rate of bioavailability, estimated to be somewhere around <1%.
Side Effects
Water Retention
It’s worth noting that since MK677 promotes the secretion of GH, one may notice that they retain more water than normal [41]. This is an often reported complaint anecdotally and is completely normal. However, one may not desire this effect from a cosmetic perspective.
Increased Hunger
MK677 is certainly not best to use when cutting due to its potent effects on ghrelin. Remember, ghrelin is the “hunger hormone”, therefore, you’ll most likely experience a dramatic increase in hunger when taking this. Therefore, MK677 is best reserved for bulking cycles. If you have trouble putting on weight, this compound will especially be helpful.
Lethargy
MK677 was often taken before bedtime in clinical trials. It could be hypothesized that the reason behind taking it before bedtime is that GH therapy is often associated with excessive sleepiness and since MK677 increases GH levels, it may lead to a person feeling significantly more fatigued than they are accustomed to during the daytime [42].
Vivid Dreams
MK677 had a significant effect on both young and old individuals on the length of their REM sleep cycles [36]. It could be possible that you’ll experience more vivid dreams as a result of using MK677, since dreaming most often takes place during the REM stage of sleep.
Dosage
The dose that was most commonly used in clinical trials was 25mg/day. This dose promoted the increase of GH and IGF-1 in most of the subjects. This dose also appears to be used quite commonly in anecdotal reports.
Should I Use a Post-Cycle Therapy (PCT) After My MK677 Cycle?
It’s not necessary to implement a PCT after using MK677, as it is not a SARM and doesn’t cause any of the side effects typically associated with SARMs or steroids such as testosterone suppression and male-pattern baldness.
References
- Chapman, I. M., Bach, M. A., Van Cauter, E., Farmer, M., Krupa, D., Taylor, A. M., Schilling, L. M., Cole, K. Y., Skiles, E. H., Pezzoli, S. S., Hartman, M. L., Veldhuis, J. D., Gormley, G. J., & Thorner, M. O. (1996). Stimulation of the growth hormone (GH)-insulin-like growth factor I axis by daily oral administration of a GH secretogogue (MK-677) in healthy elderly subjects. The Journal of clinical endocrinology and metabolism, 81(12), 4249–4257. https://doi.org/10.1210/jcem.81.12.8954023
- Smith, R. G., Sun, Y., Jiang, H., Albarran-Zeckler, R., & Timchenko, N. (2007). Ghrelin receptor (GHS-R1A) agonists show potential as interventive agents during aging. Annals of the New York Academy of Sciences, 1119, 147–164. https://doi.org/10.1196/annals.1404.023
- Ahmad, A. M., Thomas, J., Clewes, A., Hopkins, M. T., Guzder, R., Ibrahim, H., Durham, B. H., Vora, J. P., & Fraser, W. D. (2003). Effects of growth hormone replacement on parathyroid hormone sensitivity and bone mineral metabolism. The Journal of clinical endocrinology and metabolism, 88(6), 2860–2868. https://doi.org/10.1210/jc.2002-021787
- Velloso C. P. (2008). Regulation of muscle mass by growth hormone and IGF-I. British journal of pharmacology, 154(3), 557–568. https://doi.org/10.1038/bjp.2008.153
- Carrel, A. L., & Allen, D. B. (2000). Effects of growth hormone on adipose tissue. Journal of pediatric endocrinology & metabolism : JPEM, 13 Suppl 2, 1003–1009.
- Müller, T. D., Nogueiras, R., Andermann, M. L., Andrews, Z. B., Anker, S. D., Argente, J., Batterham, R. L., Benoit, S. C., Bowers, C. Y., Broglio, F., Casanueva, F. F., D’Alessio, D., Depoortere, I., Geliebter, A., Ghigo, E., Cole, P. A., Cowley, M., Cummings, D. E., Dagher, A., Diano, S., … Tschöp, M. H. (2015). Ghrelin. Molecular metabolism, 4(6), 437–460. https://doi.org/10.1016/j.molmet.2015.03.005
- Kojima, M., Hosoda, H., Date, Y., Nakazato, M., Matsuo, H., & Kangawa, K. (1999). Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature, 402(6762), 656–660. https://doi.org/10.1038/45230
- Wren, A. M., Seal, L. J., Cohen, M. A., Brynes, A. E., Frost, G. S., Murphy, K. G., Dhillo, W. S., Ghatei, M. A., & Bloom, S. R. (2001). Ghrelin enhances appetite and increases food intake in humans. The Journal of clinical endocrinology and metabolism, 86(12), 5992. https://doi.org/10.1210/jcem.86.12.8111
- Tschöp, M., Smiley, D. L., & Heiman, M. L. (2000). Ghrelin induces adiposity in rodents. Nature, 407(6806), 908–913. https://doi.org/10.1038/35038090
- Masuda, Y., Tanaka, T., Inomata, N., Ohnuma, N., Tanaka, S., Itoh, Z., Hosoda, H., Kojima, M., & Kangawa, K. (2000). Ghrelin stimulates gastric acid secretion and motility in rats. Biochemical and biophysical research communications, 276(3), 905–908. https://doi.org/10.1006/bbrc.2000.3568
- Tolle, V., Bassant, M. H., Zizzari, P., Poindessous-Jazat, F., Tomasetto, C., Epelbaum, J., & Bluet-Pajot, M. T. (2002). Ultradian rhythmicity of ghrelin secretion in relation with GH, feeding behavior, and sleep-wake patterns in rats. Endocrinology, 143(4), 1353–1361. https://doi.org/10.1210/endo.143.4.8712
- Weikel, J. C., Wichniak, A., Ising, M., Brunner, H., Friess, E., Held, K., Mathias, S., Schmid, D. A., Uhr, M., & Steiger, A. (2003). Ghrelin promotes slow-wave sleep in humans. American journal of physiology. Endocrinology and metabolism, 284(2), E407–E415. https://doi.org/10.1152/ajpendo.00184.2002
- Druce, M. R., Wren, A. M., Park, A. J., Milton, J. E., Patterson, M., Frost, G., Ghatei, M. A., Small, C., & Bloom, S. R. (2005). Ghrelin increases food intake in obese as well as lean subjects. International journal of obesity (2005), 29(9), 1130–1136. https://doi.org/10.1038/sj.ijo.0803001
- Jerlhag, E., Egecioglu, E., Dickson, S. L., Andersson, M., Svensson, L., & Engel, J. A. (2006). Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addiction biology, 11(1), 45–54. https://doi.org/10.1111/j.1369-1600.2006.00002.x
- Arvat, E., Di Vito, L., Broglio, F., Papotti, M., Muccioli, G., Dieguez, C., Casanueva, F. F., Deghenghi, R., Camanni, F., & Ghigo, E. (2000). Preliminary evidence that Ghrelin, the natural GH secretagogue (GHS)-receptor ligand, strongly stimulates GH secretion in humans. Journal of endocrinological investigation, 23(8), 493–495. https://doi.org/10.1007/BF03343763
- Peino, R., Baldelli, R., Rodriguez-Garcia, J., Rodriguez-Segade, S., Kojima, M., Kangawa, K., Arvat, E., Ghigo, E., Dieguez, C., & Casanueva, F. F. (2000). Ghrelin-induced growth hormone secretion in humans. European journal of endocrinology, 143(6), R11–R14. https://doi.org/10.1530/eje.0.143r011
- Veldhuis, J. D., Reynolds, G. A., Iranmanesh, A., & Bowers, C. Y. (2008). Twenty-four hour continuous ghrelin infusion augments physiologically pulsatile, nycthemeral, and entropic (feedback-regulated) modes of growth hormone secretion. The Journal of clinical endocrinology and metabolism, 93(9), 3597–3603. https://doi.org/10.1210/jc.2008-0620
- Lee, J., Kwon, A., Chae, H. W., Lee, W. J., Kim, T. H., & Kim, H. S. (2018). Effect of the Orally Active Growth Hormone Secretagogue MK-677 on Somatic Growth in Rats. Yonsei medical journal, 59(10), 1174–1180. https://doi.org/10.3349/ymj.2018.59.10.1174
- Jacks, T., Smith, R., Judith, F., Schleim, K., Frazier, E., Chen, H., Krupa, D., Hora, D., Jr, Nargund, R., Patchett, A., & Hickey, G. (1996). MK-0677, a potent, novel, orally active growth hormone (GH) secretagogue: GH, insulin-like growth factor I, and other hormonal responses in beagles. Endocrinology, 137(12), 5284–5289. https://doi.org/10.1210/endo.137.12.8940347
- Yakar, S., Rosen, C. J., Beamer, W. G., Ackert-Bicknell, C. L., Wu, Y., Liu, J. L., Ooi, G. T., Setser, J., Frystyk, J., Boisclair, Y. R., & LeRoith, D. (2002). Circulating levels of IGF-1 directly regulate bone growth and density. The Journal of clinical investigation, 110(6), 771–781. https://doi.org/10.1172/JCI15463
- Clemmons D. R. (2012). Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinology and metabolism clinics of North America, 41(2), 425–viii. https://doi.org/10.1016/j.ecl.2012.04.017
- Bikle, D. D., Tahimic, C., Chang, W., Wang, Y., Philippou, A., & Barton, E. R. (2015). Role of IGF-I signaling in muscle bone interactions. Bone, 80, 79–88. https://doi.org/10.1016/j.bone.2015.04.036
- Wells, T., & Houston, P. A. (2001). Skeletal growth acceleration with growth hormone secretagogues in transgenic growth retarded rats: pattern-dependent effects and mechanisms of desensitization. Journal of neuroendocrinology, 13(6), 496–504. https://doi.org/10.1046/j.1365-2826.2001.00661.x
- McDowell, R. S., Elias, K. A., Stanley, M. S., Burdick, D. J., Burnier, J. P., Chan, K. S., Fairbrother, W. J., Hammonds, R. G., Ingle, G. S., Jacobsen, N. E., Mortensen, D. L., Rawson, T. E., Won, W. B., Clark, R. G., & Somers, T. C. (1995). Growth hormone secretagogues: characterization, efficacy, and minimal bioactive conformation. Proceedings of the National Academy of Sciences of the United States of America, 92(24), 11165–11169. https://doi.org/10.1073/pnas.92.24.11165
- Copinschi, G., Van Onderbergen, A., L’Hermite-Balériaux, M., Mendel, C. M., Caufriez, A., Leproult, R., Bolognese, J. A., De Smet, M., Thorner, M. O., & Van Cauter, E. (1996). Effects of a 7-day treatment with a novel, orally active, growth hormone (GH) secretagogue, MK-677, on 24-hour GH profiles, insulin-like growth factor I, and adrenocortical function in normal young men. The Journal of clinical endocrinology and metabolism, 81(8), 2776–2782. https://doi.org/10.1210/jcem.81.8.8768828
- Veldhuis, J. D., Liem, A. Y., South, S., Weltman, A., Weltman, J., Clemmons, D. A., Abbott, R., Mulligan, T., Johnson, M. L., & Pincus, S. (1995). Differential impact of age, sex steroid hormones, and obesity on basal versus pulsatile growth hormone secretion in men as assessed in an ultrasensitive chemiluminescence assay. The Journal of clinical endocrinology and metabolism, 80(11), 3209–3222. https://doi.org/10.1210/jcem.80.11.7593428
- de Boer, H., Blok, G. J., & Van der Veen, E. A. (1995). Clinical aspects of growth hormone deficiency in adults. Endocrine reviews, 16(1), 63–86. https://doi.org/10.1210/edrv-16-1-63
- Björntorp P. (1993). Visceral obesity: a “civilization syndrome”. Obesity research, 1(3), 206–222. https://doi.org/10.1002/j.1550-8528.1993.tb00614.x
- Svensson, J., Lönn, L., Jansson, J. O., Murphy, G., Wyss, D., Krupa, D., Cerchio, K., Polvino, W., Gertz, B., Boseaus, I., Sjöström, L., & Bengtsson, B. A. (1998). Two-month treatment of obese subjects with the oral growth hormone (GH) secretagogue MK-677 increases GH secretion, fat-free mass, and energy expenditure. The Journal of clinical endocrinology and metabolism, 83(2), 362–369. https://doi.org/10.1210/jcem.83.2.4539
- Christoffersen, C. T., Bornfeldt, K. E., Rotella, C. M., Gonzales, N., Vissing, H., Shymko, R. M., ten Hoeve, J., Groffen, J., Heisterkamp, N., & De Meyts, P. (1994). Negative cooperativity in the insulin-like growth factor-I receptor and a chimeric IGF-I/insulin receptor. Endocrinology, 135(1), 472–475. https://doi.org/10.1210/endo.135.1.8013387
- Svensson, J., Carlsson, B., Carlsson, L. M., Jansson, J. O., & Bengtsson, B. A. (1999). Discrepancy between serum leptin values and total body fat in response to the oral growth hormone secretagogue MK-677. Clinical endocrinology, 50(4), 451–456. https://doi.org/10.1046/j.1365-2265.1999.00667.x
- Bowers, C. Y., Reynolds, G. A., Durham, D., Barrera, C. M., Pezzoli, S. S., & Thorner, M. O. (1990). Growth hormone (GH)-releasing peptide stimulates GH release in normal men and acts synergistically with GH-releasing hormone. The Journal of clinical endocrinology and metabolism, 70(4), 975–982. https://doi.org/10.1210/jcem-70-4-975
- Mårin, P., Darin, N., Amemiya, T., Andersson, B., Jern, S., & Björntorp, P. (1992). Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism: clinical and experimental, 41(8), 882–886. https://doi.org/10.1016/0026-0495(92)90171-6
- Svensson, J., Ohlsson, C., Jansson, J. O., Murphy, G., Wyss, D., Krupa, D., Cerchio, K., Polvino, W., Gertz, B., Baylink, D., Mohan, S., & Bengtsson, B. A. (1998). Treatment with the oral growth hormone secretagogue MK-677 increases markers of bone formation and bone resorption in obese young males. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research, 13(7), 1158–1166. https://doi.org/10.1359/jbmr.1998.13.7.1158
- Murphy, M. G., Bach, M. A., Plotkin, D., Bolognese, J., Ng, J., Krupa, D., Cerchio, K., & Gertz, B. J. (1999). Oral administration of the growth hormone secretagogue MK-677 increases markers of bone turnover in healthy and functionally impaired elderly adults. The MK-677 Study Group. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research, 14(7), 1182–1188. https://doi.org/10.1359/jbmr.1999.14.7.1182
- Copinschi, G., Leproult, R., Van Onderbergen, A., Caufriez, A., Cole, K. Y., Schilling, L. M., Mendel, C. M., De Lepeleire, I., Bolognese, J. A., & Van Cauter, E. (1997). Prolonged oral treatment with MK-677, a novel growth hormone secretagogue, improves sleep quality in man. Neuroendocrinology, 66(4), 278–286. https://doi.org/10.1159/000127249
- Rasch, B., & Born, J. (2013). About sleep’s role in memory. Physiological Reviews, 93(2), 681-766. https://doi.org/10.1152/physrev.00032.2012
- Shankar, G. M., Li, S., Mehta, T. H., Garcia-Munoz, A., Shepardson, N. E., Smith, I., Brett, F. M., Farrell, M. A., Rowan, M. J., Lemere, C. A., Regan, C. M., Walsh, D. M., Sabatini, B. L., & Selkoe, D. J. (2008). Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nature medicine, 14(8), 837–842. https://doi.org/10.1038/nm1782
- Sevigny, J. J., Ryan, J. M., van Dyck, C. H., Peng, Y., Lines, C. R., Nessly, M. L., & MK-677 Protocol 30 Study Group (2008). Growth hormone secretagogue MK-677: no clinical effect on AD progression in a randomized trial. Neurology, 71(21), 1702–1708. https://doi.org/10.1212/01.wnl.0000335163.88054.e7
- Patchett, A. A., Nargund, R. P., Tata, J. R., Chen, M. H., Barakat, K. J., Johnston, D. B., Cheng, K., Chan, W. W., Butler, B., & Hickey, G. (1995). Design and biological activities of L-163,191 (MK-0677): a potent, orally active growth hormone secretagogue. Proceedings of the National Academy of Sciences of the United States of America, 92(15), 7001–7005. https://doi.org/10.1073/pnas.92.15.7001
- Møller, J., Nielsen, S., & Hansen, T. K. (1999). Growth hormone and fluid retention. Hormone research, 51 Suppl 3, 116–120. https://doi.org/10.1159/000053173
- Gohil, A., & Eugster, E. (2019). Growth Hormone Deficiency and Excessive Sleepiness: A Case Report and Review of the Literature. Pediatric endocrinology reviews : PER, 17(1), 41–46. https://doi.org/10.17458/per.vol17.2019.ge.ghdeficiencyandsleepiness
