Ostarine MK2866: The Complete Guide
*DISCLAIMER: Selective Androgen Receptor Modulators (SARMs) are not approved by the FDA for any currently acceptable medicinal/therapeutic purpose. Therefore, this article does not serve as medical advice and is not to be intended to be used to diagnose, treat, cure or prevent any disease. SARMS are to be used for research purposes only and not intended for human consumption.*
What is Ostarine?
Ostarine, also known as Enobosarm, MK-2866, and GTx-024, could be presumed to be the most extensively researched selective androgen receptor modulator (SARM) to date. There has been more data collected on Ostarine compared to any other SARM thus far.
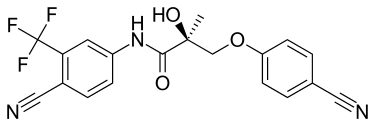
Ostarine was originally developed as a treatment for muscle-wasting diseases (cachexia). Common examples of cachexia include cancer, rheumatoid arthritis, and congestive heart failure .
Other areas in which Ostarine’s effects are being investigated include age-related muscle loss (sarcopenia) and the post-menopausal period in women, with the latter often resulting in both loss of bone density and skeletal muscle tissue.
Anabolic-androgenic steroids (AAS), such as testosterone and its various derivatives, are used to treat these aforementioned conditions. AAS has proven to be effective for these conditions, facilitating patients to regain the lean body mass (LBM) they had lost from their pathology [1]. But therein lies a problem with AAS treatment (androgen therapy); its lack of ability to exert effects only on the bodily areas of interest. Adverse events arise when therapy begins to target unintended regions. This is the foundation amongst which Ostarine was initially developed.
Androgen Therapy
In order to understand the potential for Ostarine to replace traditional androgen treatment, it’s imperative to consider the relationship that endogenous testosterone production has on male bodily development and functioning. Testosterone produced within the body has profound influence over the synthesis of 2 key enzymes: 5α-reductase (“α” is pronounced “alpha”) and aromatase.
5α-Reductase
This enzyme is responsible for converting testosterone into dihydrotestosterone (DHT). DHT binds to the androgen receptor (AR) at ~2-3x greater affinity than testosterone [2]. In other words, it binds to the AR with double, or perhaps even triple the strength that testosterone normally would. As a result, DHT has the propensity to be up to 10x more potent as a result.
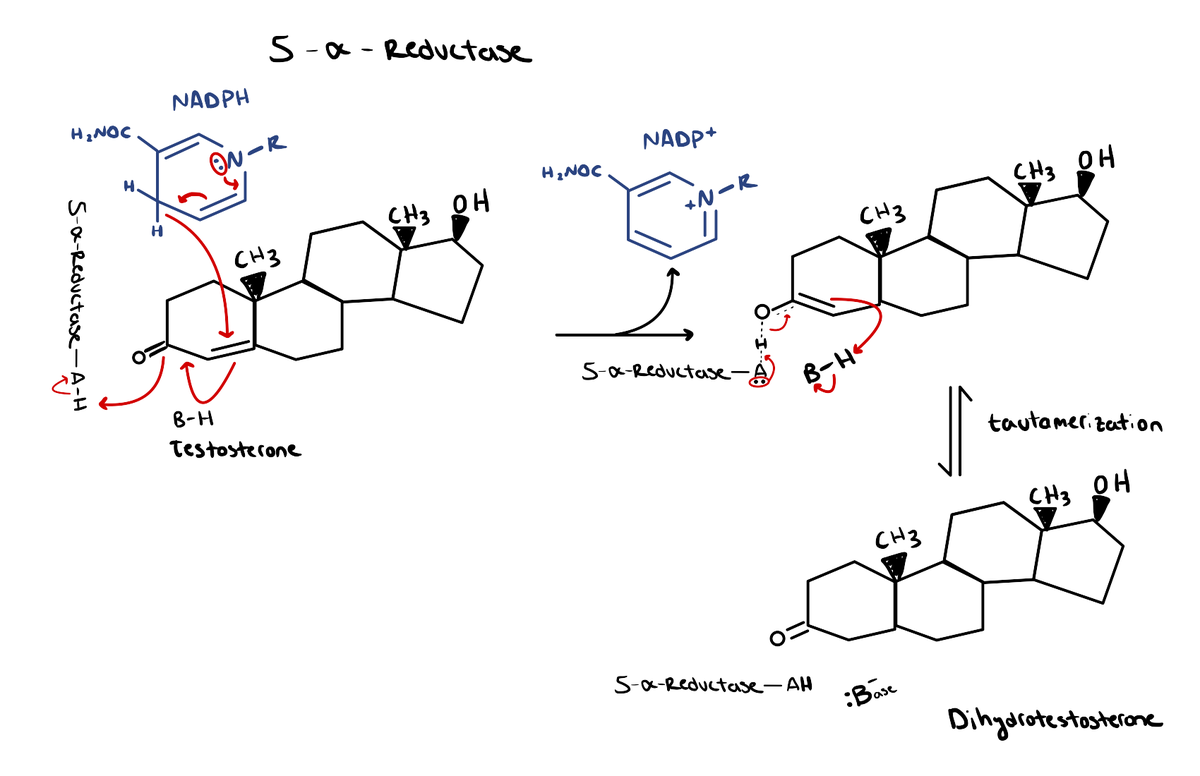
DHT is instrumental in the development of male sexual characteristics during childhood and adolescence, such as facial/body hair, penis/testicle maturation, and prostate growth. During adulthood, though, it plays more of a pathological role.
DHT-related conditions can be related to both abnormally high or low levels of DHT in the body. Conditions that commonly result from excessively high DHT levels are male-pattern baldness (androgenic alopecia) and prostate enlargement, the latter of which can cause benign prostate hypertrophy (BPH) and prostate cancer.
Low levels of DHT would cause ultimately cause a state of androgen deficiency, resulting in the onset of various undesirable symptoms, such as:
- Loss of libido
- Infertility
- Decreased muscle mass
- Increased fat mass
- Fatigue
- Anxiety/Depression
Aromatase
Aromatase is the enzyme responsible for converting testosterone into estrogen, the female sex steroid. As with DHT, both excessively high or low levels of estrogen often result in the onset of undesirable signs and symptoms.
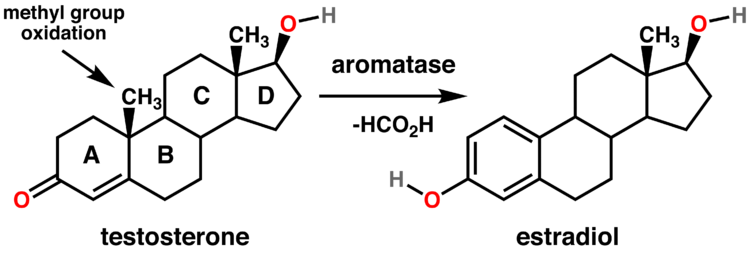
While some men may believe that lower levels of estrogen in the body would be better than having estrogen that falls within normal ranges, this is simply not the case. Men need a particular amount of estrogen in their bodies in order to maintain normal bodily functioning.
Low estrogen levels in men will primarily lead to bone resorption; the breakdown of bone tissue and the release of the minerals contained within it (i.e. calcium) into circulation [3]. This ultimately leads to lower bone density, naturally limiting one’s capacity to perform any form of strength training, especially when lifting heavier loads.
Symptoms that can also indicate low estrogen levels in men include:
- Fatigue
- Anxiety
- Erectile dysfunction (ED)
- Loss of bone density
- Mood swings
- Appetite loss
As for symptoms of high estrogen, these include:
- Loss of muscle mass
- Fatigue
- Loss of libido
- Gynecomastia (development of excess breast tissue)
- Depression
- Irritability
The potential for these types of issues to arise alongside androgen therapy can all be traced back to the current circulating levels of testosterone in the body. This is due to its influence over the vast hormonal cascade of events involving DHT and estrogen.
Ostarine as a Potential Replacement for Testosterone
This is where a SARM like Ostarine would enter the picture, due to its nonsteroidal nature. The primary goal when developing Ostarine was to be able to target only the desired tissue (i.e. skeletal muscle, bone tissue), while simultaneously avoiding these same effects on tissues that are not attempted to be targeted (e.g. prostate, heart). Ostarine doesn’t go through 5α-reduction or aromatization either, meaning that it will not convert into either DHT or estrogen, respectively.
But there are drawbacks. Since Ostraine does not aromatize into estrogen, it may lead to symptoms of low estrogen, as previously discussed. It may appear contradictory, but Ostarine may be able to cause symptoms of high estrogen in men as well. This at least appears to be a possibility at the onset of treatment. When treatment is started, the patient will still have their baseline levels of testosterone circulating in their bloodstream. So when Ostarine is administered, it will “compete” with testosterone for binding at the AR, potentially replacing the testosterone that would have originally been bound to the AR. Alternatively, this may result in the testosterone that was originally bound for the AR to instead be aromatized into estrogen.
Of course this is all just speculative and has not been definitively demonstrated in the form of empirical evidence thus far. But what provides Ostarine an edge over androgen therapy is its lack of 5α-reduction to DHT. Hypothetically speaking, this drastically limits and may even prevent the onset of DHT-related pathologies such as male-pattern baldness and prostate cancer.
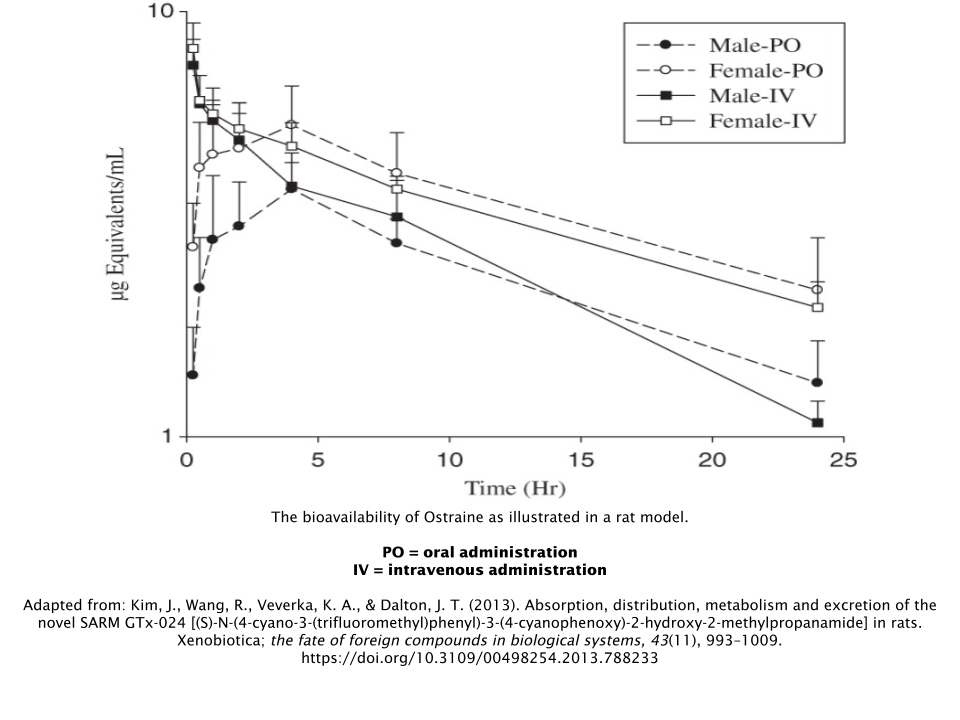
Bioavailability
Ostarine has been shown to have an overall high level of oral bioavailability [4]. This allows for stable blood levels throughout the day with everyday oral dosing. This is in contrast to the rapid spikes and falls associated with weekly/biweekly intramuscular injections or topical applications of testosterone.
A compounds’ level of selectivity for the AR is often assessed using an anabolic : androgenic ratio, or selectivity ratio. In this raio, a higher anabolic rating (number on the left) relative to the androgenic rating (number on the right) is considered to be more favorable, and vice versa.
For example, testosterone is used as a baseline for this ratio measurement, with a 1:1 ratio. While no ratio has been definitively established for Ostarine, it’s certainly higher than 1:1, and estimated to be a minimum of 10:1, which is exponentially higher than testosterone.
Human Trial Data
Phase II Trials
In 2011, a 12-week Phase-II trial was conducted on 120 elderly men and postmenopausal women in order to assess Ostarine’s effects on LBM retention, physical function (measured via stair-climbing test), body weight, and overall safety [5]. The subjects were split up into 5 different dosing groups:
- 0mg/day (placebo)
- 0.1mg/day
- 0.3mg/day
- 1mg/day
- 3mg/day
Subjects in the highest dose group (3mg/day) achieved a LBM increase of 1.3kg (2.87lbs), while simultaneously losing 0.6kg (1.32lbs) of body fat. During the stair-climbing test, researchers observed that the group taking the highest dose of 3mg/day had the greatest levels of improvement.
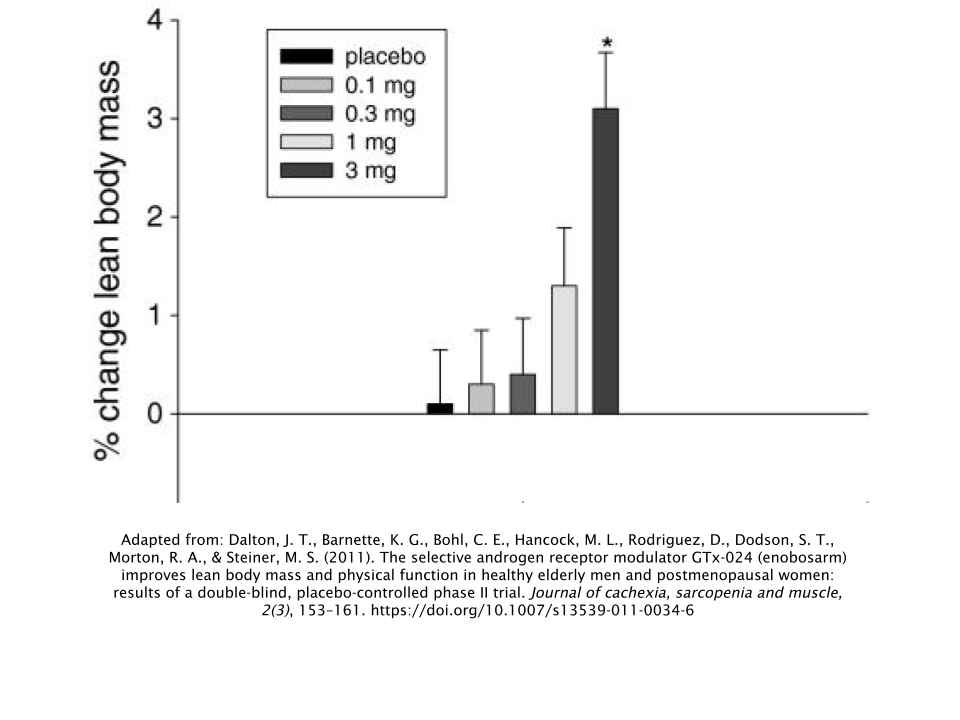
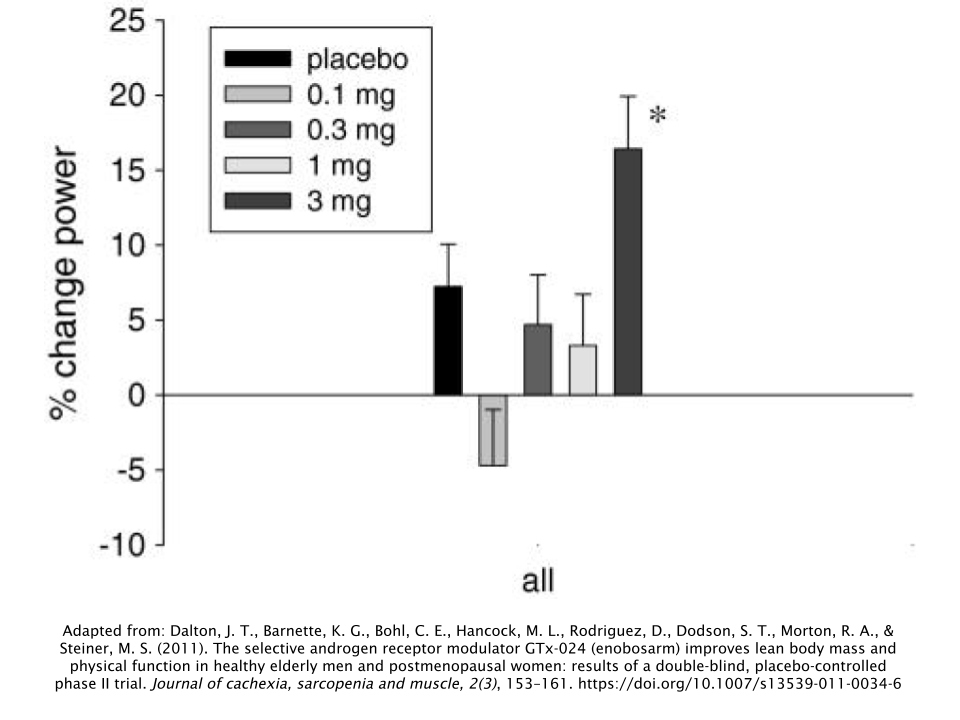
The men did not experience any changes in free testosterone, LH, or follicle-stimulating hormone (FSH). But the men taking 1mg/day and 3mg/day of Ostarine (the two highest-dose groups) did have significant decreases in their levels of both sex-hormone binding globulin (SHBG) and total testosterone.
SHBG is responsible for inhibiting androgens such as testosterone and DHT from exerting their respective effects throughout the body. Levels of free testosterone are often indicative of SHBG levels, as only 0.5% – 3% of testosterone in men is in an unbound, or free state [6]. So it’s puzzling why free testosterone levels were not significantly affected. Perhaps a higher dosage is needed to cause such an effect.
There was a 61% decrease in SHBG observed in the group that took the highest treatment dose of Ostarine at 3mg/day. On the other hand, men treated with 600mg/week of testosterone, which is ~6x greater than a therapeutic hormone replacement dose, only observed a 31% decrease in SHBG [7].
Another 12-week Phase II study, this time with 88 postmenopausal women, demonstrated significant increases in both LBM and leg strength in those who took 3mg/day of Ostarine [8]. In addition, there were no notable effects on the women’s uterus’, as well as no modification to their liver enzymes.
This was a noteworthy discovery, as providing androgen therapy to women has always been a major concern due to the high risk of the onset of virilization, or masculinization, symptoms in women. There were no effects observed on the uterus of the subjects; demonstrating Ostarine’s ability to exert selective actions on the AR. This is in contrast to commonly prescribed androgen therapies for women, such as Oxandrolone (Anavar™) [9].
Phase III Trials
Ostarine is the only SARM to have been granted “fast track” designation status from the Food & Drug Administration (FDA). This process helps to expedite the drug testing process in order to treat serious conditions in high demand of effective pharmacological treatments.
GTx, Inc., the company that manufactures Ostarine, was granted this status from the FDA and as a result, created the POWER trials; two identically-designed Phase III trials conducted in various medical facilities around the United States. These trials aimed to examine a condition called non-small cell lung cancer (NSCLC) [10,11]. In these trials, 325 subjects were given either placebo or 3mg/day of Ostarine for 12 weeks and were primarily assessed on physical function (stair climb test) and changes in LBM. Secondarily, the researchers examined any differences in the quality of life and overall healthcare utilization of those who received Ostarine treatment versus those that did not.
GTx had announced that both trials had failed to meet the outcome goals they had made with the FDA after the trials had completed. While the average of the results from both trials indicated a failure overall, Ostarine was able to continue following the trend it had set in previous studies in regards to its positive effects on LBM.
How Does Ostarine Compare to Other SARMS?
Ostarine has been included in the same study design as a couple of other SARMS, those being LGD-4033 and Andarine (S4).
Ligandrol (LGD-4033)
In a male rat model for hypogonadism, Ligandrol was shown to have significantly greater selectivity for the androgen receptor compared to Ostarine; with Ligandrol having 500x the selectivity of testosterone and Ostarine having only 390x the selectivity of testosterone [12].
This is illustrated by administration of Ligandrol resulting in greater rates of muscle accretion, with similar effects on the prostate.
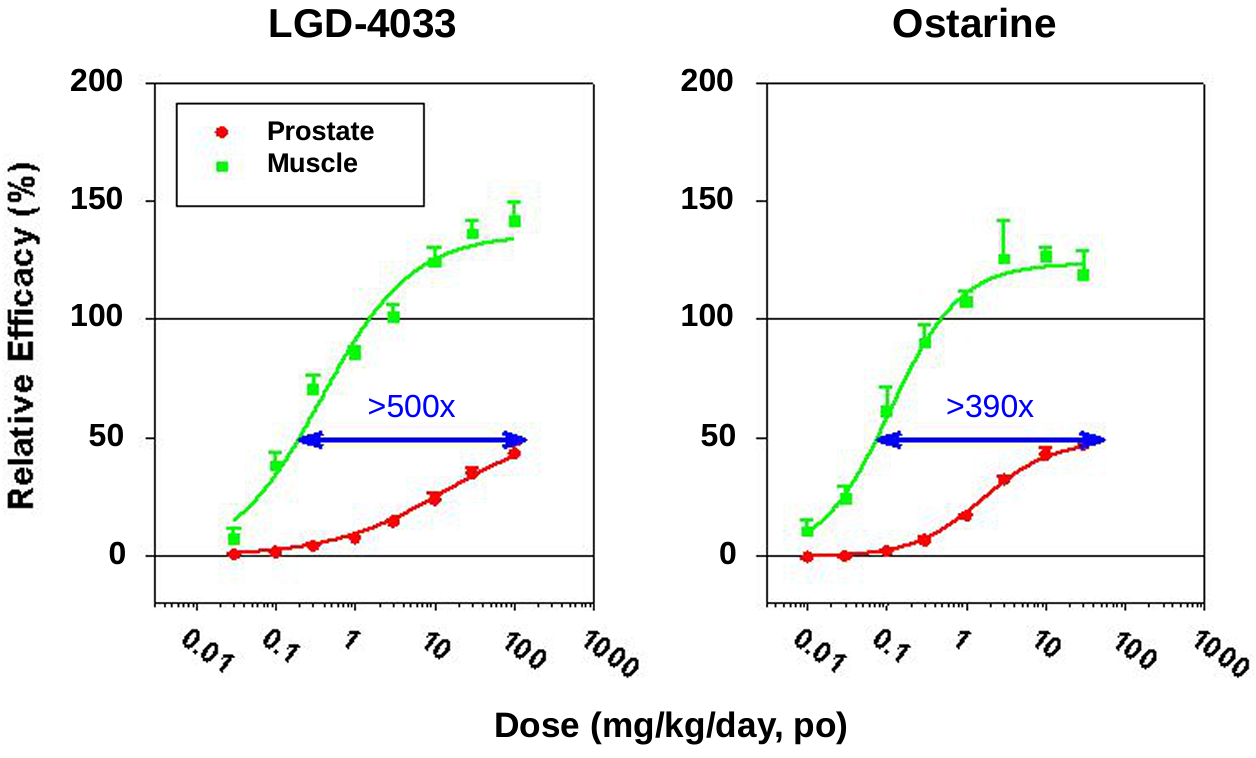
Adapted from: Higgins, John. June 24, 2010. Investor and Analyst Day, Exhibit 99.1. Ligand Pharmaceuticals, Inc. https://www.sec.gov/Archives/edgar/data/886163/000119312510146054/dex991.htm
Since Ligandrol is considered to be a bulking compound amongst recreational users, it would make sense that it would have a stronger anabolic effect than Ostarine. While not supported by any currently available data, it could be postulated that the increased anabolic activity could cause greater levels of testosterone suppression. Therefore, recovery from an Ligandrol cycle could prove to be more difficult than a milder cycle with Ostarine if this were to be proven true later on down the line.
Andarine (S4)
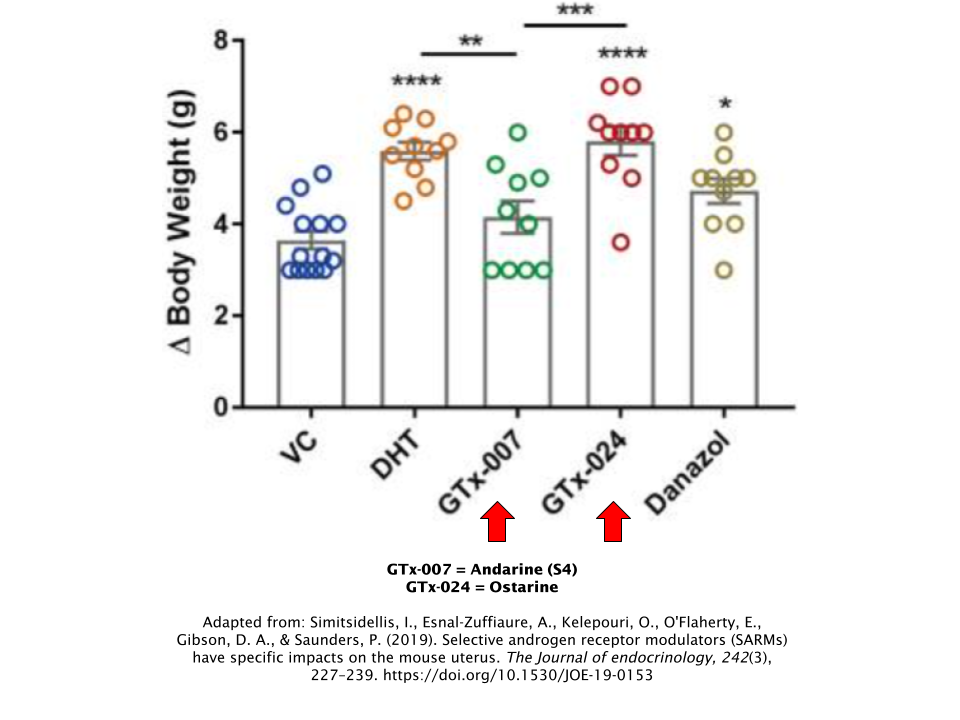
Andarine was formerly produced by GTx Inc., the same company that currently makes Ostarine and was essentially the predecessor to Ostarine. However, they abandoned data collection after preclinical animal trials, so no human data is available on Andarine. Therefore, it’s difficult to make any direct comparisons between Ostarine and Andarine.
However, preclinical data conducted on mice that involved both of these SARMs suggests that Ostarine is the clear winner here in terms of overall anabolic potency [13]. Andarine had failed to increase body weight, among other parameters, whereas Ostarine had succeeded.
Side Effects/Safety
Ostarine has been shown to be well-tolerated, both clinically and anecdotally. During clinical trials, it appears that Ostarine is well-tolerated by an overwhelmingly large majority of subjects, up to a dose of 3mg [14]. This dose was established during a Phase II trial in cancer patients, which appeared to have influenced GTx to use it in their POWER trials thereafter.
Despite its high tolerability, there are still side effects that have presented themselves in the scientific literature that are worth noting.
Cholesterol
In the aforementioned Phase II trial involving the 88 postmenopausal women, Ostarine had lowered the HDL (“good” cholesterol) of the treatment group by 24% [8]. Studies have previously demonstrated that Ostarine reduces HDL cholesterol in a dose-dependent fashion (meaning that the level of HDL suppression increases as the dose increases, and vice versa). But the majority of the data suggests that the ratio of subject’s HDL to LDL, or “bad” cholesterol only reaches the lowest risk category cardiovascular risk classification [15].
Liver Toxicity (Hepatotoxicity)
Ostarine does not appear to negatively impact liver enzymes in studies that had examined them. In one of Ostarine’s Phase II clinical trials, 8 of the 120 subjects demonstrated clinically significant elevations of the liver enzyme alanine transaminase (ALT). Fortunately, this issue had resolved as the subjects continued to take their continued daily dosage of 3mg/day with the exception of 1 subject, who was eventually withdrawn from the study [5].
Testosterone Suppression
Research on Ostarine has demonstrated its ability to decrease total testosterone in a dose-dependent fashion at 1mg/day and 3mg/day doses, indicating that doses above 1mg/day carry a much greater risk of causing testosterone suppression than doses below that threshold [5].
Assuming that those aiming to use Ostarine for recreational purposes will take doses far exceeding 3mg/day, one could hypothesize that greater levels of testosterone suppression would occur as the dosage continues to climb.
Dosing
It has been reported that Ostarine has a half-life of approximately 1 day, making it suitable for simple, once-a-day oral dosing protocol [13].
Ostarine is quite versatile in nature, as it is suitable for both bulking (mass gain) and cutting (fat loss). Anecdotal reports traditionally suggest doses between 10mg/day – 25mg/day and are generally well-tolerated. To err on the side of safety, always begin your cycle with the lowest dose and work your way up if you begin to observe any plateaus.
The goal in a more conservative approach like this is to find the minimum effective dose, meaning the lowest dose possible that still achieves the objectives of taking the drug in the first place. Remember, more is not always better! As with most drugs (anabolic compounds in particular), there is a law of diminishing returns, meaning that the dose can only be so high before greater side effects/adverse events are observed, while at the same time not amplifying the desired effect to any notable extent.
Post-Cycle Therapy (PCT)
It’s highly recommended that you complete a PCT after an Ostarine cycle due to its ability to suppress total testosterone levels. The half-life of Ostarine is approximately 24 hours, therefore PCT should begin the day after your last dose.
It’s not necessary to include aromatase inhibitors in your PCT. Using a selective estrogen receptor modulator (SERM) should suffice; the two most popular choices being either tamoxifen (Nolvadex®) or clomiphene (Clomid®). While using tamoxifen (Nolvadex®) is recommended due to its milder nature, some prefer the use of clomiphene (Clomid®). Either way, one or the other should be just fine. Rarely is it necessary to take both of these SERMs simultaneously in order to recover properly.
No matter which one you choose, here’s what a simple 4-week PCT protocol would look like for these SERMS:
|
Week |
Tamoxifen (Nolvadex®) |
OR |
Clomiphene (Clomid®) |
|
1 |
40 mg/day |
50 mg/day |
|
|
2 |
40 mg/day |
50 mg/day |
|
|
3 |
20 mg/day |
25 mg/day |
|
|
4 |
20 mg/day |
25 mg/day |
References
- Gullett, N. P., Hebbar, G., & Ziegler, T. R. (2010). Update on clinical trials of growth factors and anabolic steroids in cachexia and wasting. The American journal of clinical nutrition, 91(4), 1143S–1147S. https://doi.org/10.3945/ajcn.2010.28608E
- Mozayani. (2007). Handbook of drug interactions: A clinical and forensic guide.
- Teitelbaum S. L. (2000). Bone resorption by osteoclasts. Science (New York, N.Y.), 289(5484), 1504–1508. https://doi.org/10.1126/science.289.5484.1504
- Kim, J., Wang, R., Veverka, K. A., & Dalton, J. T. (2013). Absorption, distribution, metabolism and excretion of the novel SARM GTx-024 [(S)-N-(4-cyano-3-(trifluoromethyl)phenyl)-3-(4-cyanophenoxy)-2-hydroxy-2-methylpropanamide] in rats. Xenobiotica; the fate of foreign compounds in biological systems, 43(11), 993–1009. https://doi.org/10.3109/00498254.2013.788233
- Dalton, J. T., Barnette, K. G., Bohl, C. E., Hancock, M. L., Rodriguez, D., Dodson, S. T., Morton, R. A., & Steiner, M. S. (2011). The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled Phase II trial. Journal of cachexia, sarcopenia and muscle, 2(3), 153–161. https://doi.org/10.1007/s13539-011-0034-6
- Belchetz, P. E., Barth, J. H., & Kaufman, J. M. (2010). Biochemical endocrinology of the hypogonadal male. Annals of clinical biochemistry, 47(Pt 6), 503–515. https://doi.org/10.1258/acb.2010.010150
- Bhasin, S., Storer, T. W., Berman, N., Callegari, C., Clevenger, B., Phillips, J., Bunnell, T. J., Tricker, R., Shirazi, A., & Casaburi, R. (1996). The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. The New England journal of medicine, 335(1), 1–7. https://doi.org/10.1056/NEJM199607043350101
- Marcantonio, E. E., Witter, R. E., Ding, Y., Xu, Y., Klappenbach, J., Wang, Y., Wong, P. H., Liu, F., Chodakewitz, J., Wagner, J. A., & Stoch, S. A. (2010). A 12-Week pharmacokinetic and Pharmacodynamic study of two selective androgen receptor modulators (SARMs) in postmenopausal subjects. Posters I, P2-2-P2-2. https://doi.org/10.1210/endo-meetings.2010.part2.p1.p2-2
- Freriks, K., Sas, T. C., Traas, M. A., Netea-Maier, R. T., den Heijer, M., Hermus, A. R., Wit, J. M., van Alfen-van der Velden, J. A., Otten, B. J., de Muinck Keizer-Schrama, S. M., Gotthardt, M., Dejonckere, P. H., Zandwijken, G. R., Menke, L. A., & Timmers, H. J. (2012). Long-term effects of previous oxandrolone treatment in adult women with Turner syndrome. European journal of endocrinology, 168(1), 91–99. https://doi.org/10.1530/EJE-12-0404
- Phase III study of the effect of gtx-024 on muscle wasting in patients with non-small cell lung cancer (NSCLC). (n.d.). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT01355484
- Effect of gtx-024 on muscle wasting in patients with non-small cell lung cancer (NSCLC) on first line platinum. (n.d.). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/study/NCT01355497
- Higgins, John. June 24, 2010. Investor and Analyst Day, Exhibit 99.1. Ligand Pharmaceuticals, Inc. https://www.sec.gov/Archives/edgar/data/886163/000119312510146054/dex991.htm
- Simitsidellis, I., Esnal-Zuffiaure, A., Kelepouri, O., O’Flaherty, E., Gibson, D. A., & Saunders, P. (2019). Selective androgen receptor modulators (SARMs) have specific impacts on the mouse uterus. The Journal of endocrinology, 242(3), 227–239. https://doi.org/10.1530/JOE-19-0153
- Dobs, A. S., Boccia, R. V., Croot, C. C., Gabrail, N. Y., Dalton, J. T., Hancock, M. L., Johnston, M. A., & Steiner, M. S. (2013). Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. The Lancet. Oncology, 14(4), 335–345. https://doi.org/10.1016/S1470-2045(13)70055-X
- Narayanan, R., Mohler, M. L., Bohl, C. E., Miller, D. D., & Dalton, J. T. (2008). Selective androgen receptor modulators in preclinical and clinical development. Nuclear receptor signaling, 6, e010. https://doi.org/10.1621/nrs.06010
